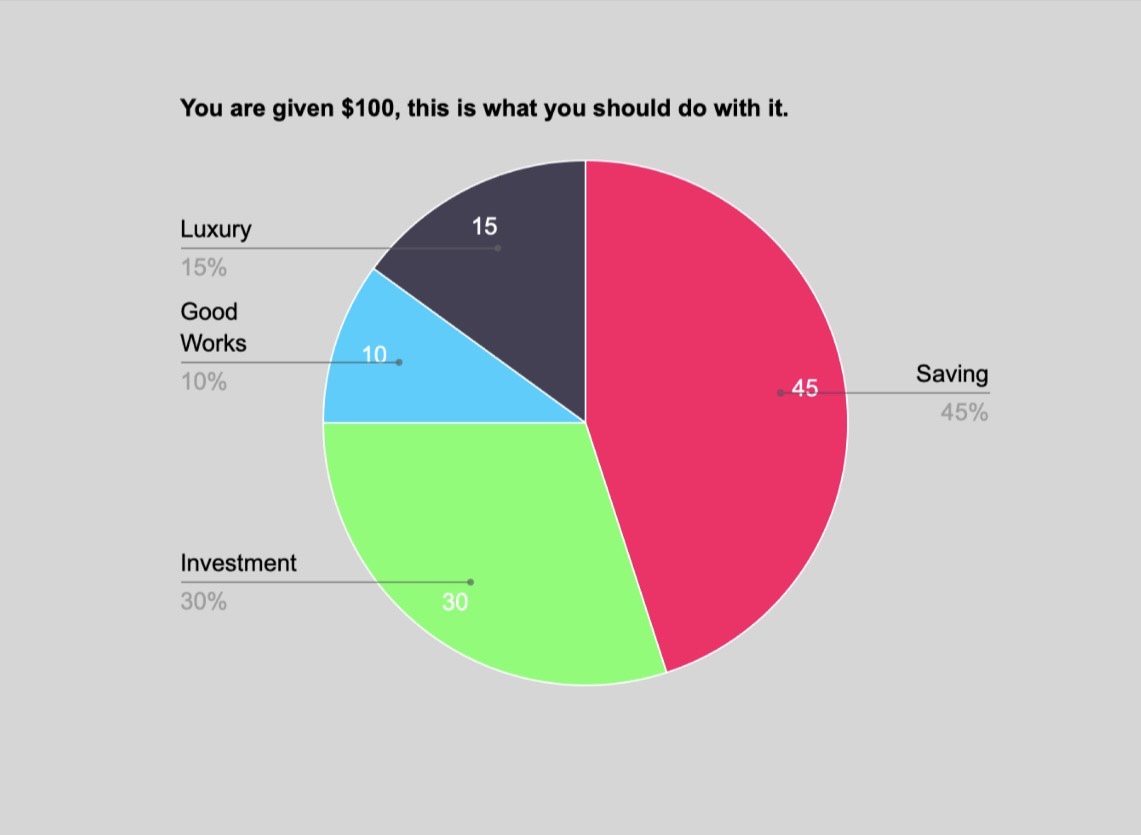
“Name the following hydrocarbon,” read the instructions on the test. Can its name be 2, 3 – Dimethylheptane. No…the teacher said it was 3 – ethyl, 2 methylheptane Or, wait. Is it 2-3 Dimethylpentane?
As I tried to finish my test, questions and more questions started appearing in my mind. What about their bonds? Are cis and trans somehow involved? And although I racked my brain
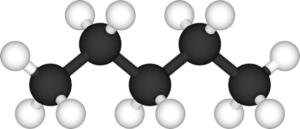
to hopefully obtain answers, none came. In the midst of my frustration, the bell rang, signaling the end of testing time. So I turned in my unfinished handout, while silently condemning “hydrocarbons.” They are of no use, solely drawings of petty sticks attached to “H” and “C.”
However, this is simply an underestimation of the actual functions that hydrocarbons have. They are not just simple drawings. In reality, they form the foundation of the energy we need. When burned, they release carbon dioxide, water, and heat. This is then used for various purposes, whether that involves heating your home or producing electricity. Without them, it would be extremely difficult for us to move around, seeing as they are major components of petroleum (“Hydrocarbon”).

Furthermore, it is necessary that we closely observe them. This is because they can also lead to negative consequences for our environment. For example, carbon dioxide, a product of the combustion of hydrocarbons, is responsible for the greenhouse effect. Hydrocarbons can damage our bodies as well. Carbon monoxide, a toxic chemical, can also be released when these are burned (“Hydrocarbon”). Therefore, hydrocarbons are necessary components of our lives that we must understand in order to protect our environment and to benefit from their functions.